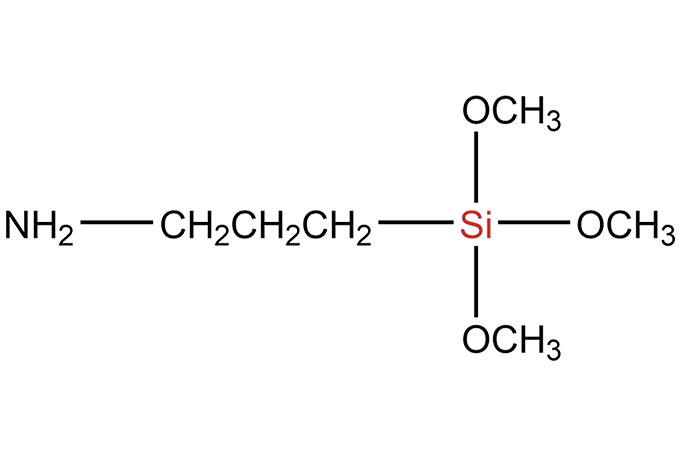
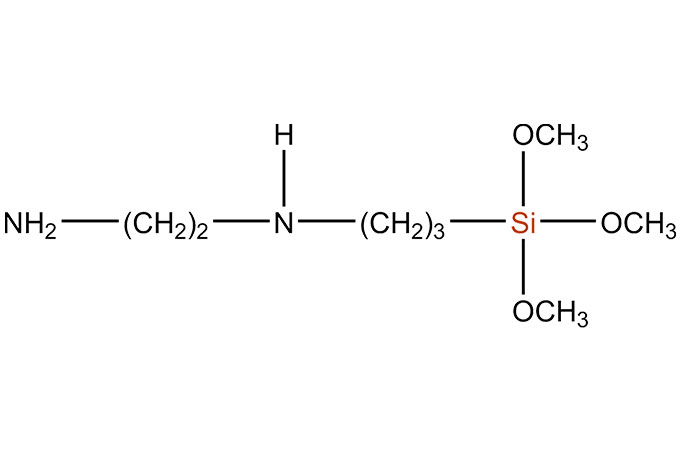
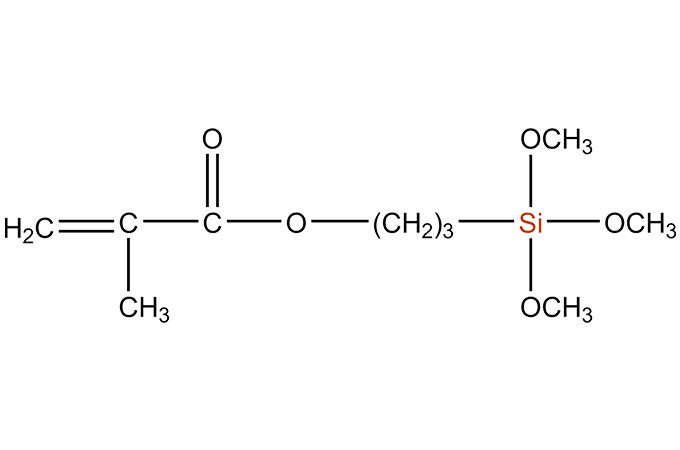
SiSiB® silane adhesion promoters are bifunctional organosilicone compounds that act as molecular bridges between the polymer matrix of an adhesive or sealant and the substrate, either inorganic or organic. SiSiB® SILANES can improve adhesive or sealant adhesion significantly. SiSiB® SILANES react chemically and promote adhesion: (1) between the base polymer in a sealant and the inorganic fillers in the formulation and/or (2) between the base polymer and the substrate.
The silane end contains hydrolysable alkoxy silane groups that are activated by reaction with ambient moisture. The hydrolysable alkoxy groups attached to the silicon end of the silane are typically either methoxy or ethoxy. Once activated (hydrolyzed), the resultant silanol groups will condense with other silanols or with reactive groups on the surface of a substrate such as SiOH, AIOH, or other metal oxides or hydroxides. The silane’s ability to bond to a surface will generally be determined by the concentration of such sites on the surface.
Selecting the optimal silane for an application requires matching the reactivity of the silane's organofunctional group to that of the polymer.
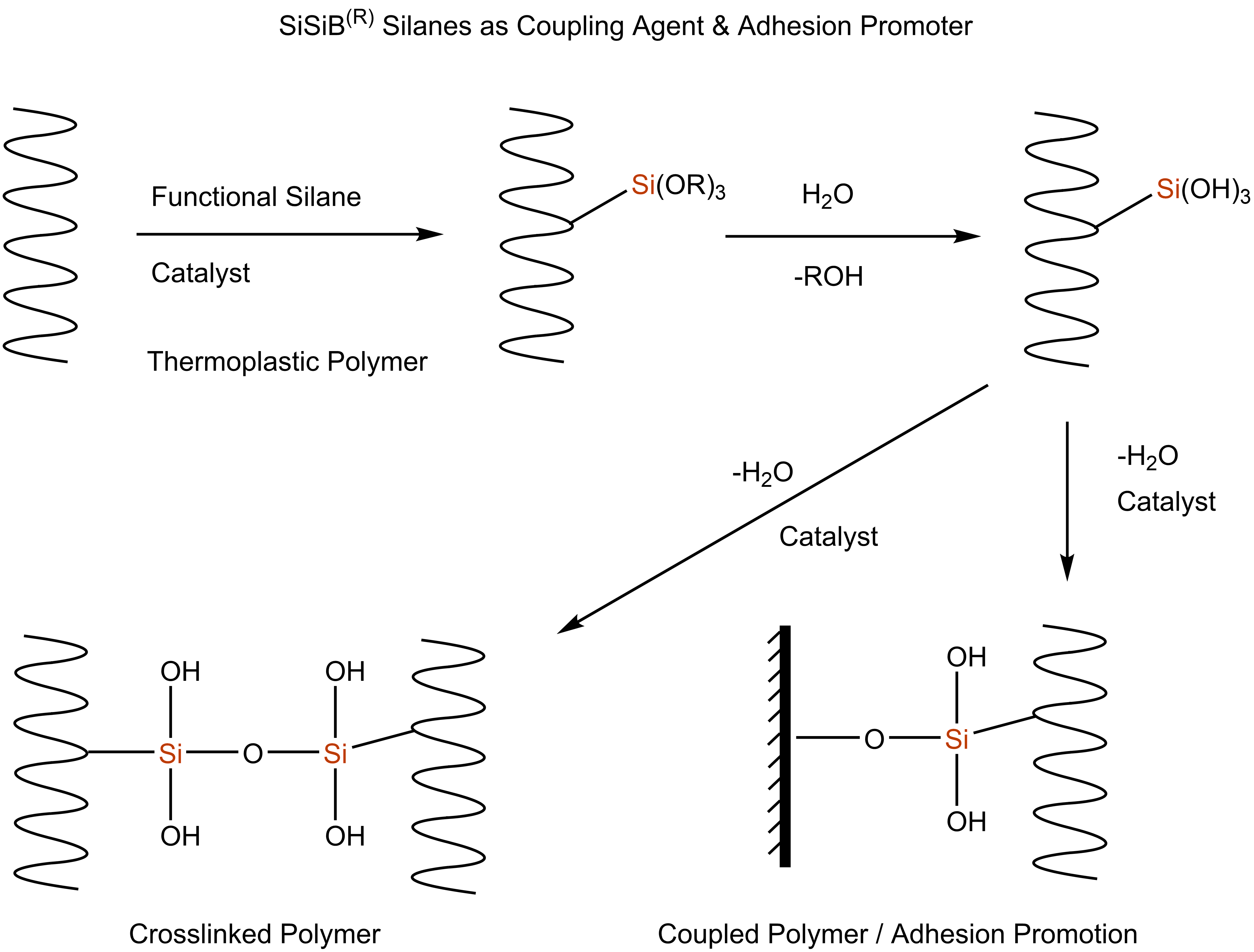
The silanes can be blended into an adhesive formulation or used as primers on substrates. The structure and reactivity of the silane will affect the ability of the silane to migrate. The most effective way to promote adhesion is to apply the silane as a primer to the surface, followed by application of the silicone adhesive/sealant. In this way, the silane will be on the surface and therefore at the interface where it can enhance adhesion between the polymer and the substrate. Silane primers are usually dilute solutions of 0.5 to 5 percent silane in alcohol or water/alcohol solvent. They are wiped or sprayed on the substrate, after which the solvent is allowed to evaporate. While the concentration needed for a specific application may vary, one percent (1%) based on resin content is recommended as a good starting point.
Excellent Effect | Silica, alumina, glass, quartz, porcelain clay |
Good Effect | Mica, Talc, clay, water and alumina, grammiteiron dust, potassium titanic acid |
Slight Effect | Asbestos, ferric oxide, zinc oxide, carborundum, silicon nitride |
Poor Effect | calcium carbonate, carbon, barium sulphate, boron |
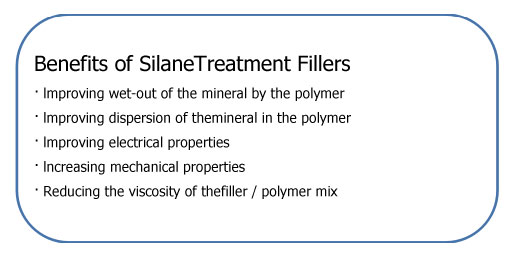
There are three general methods for treating the surfaces of inorganic filler materials before they are added to the organic resins.
a) Wet Method
By mixing slurry of the inorganic materials in a dilute solution of the silane coupling agent, a highly uniform and precise surface treatment of the inorganic material can be obtained.
b) Dry Method
A high shear, high speed, mixer is used to disperse the silane coupling agent into the inorganic materials. The silane is generally applied either neat or as a concentrated solution. When compared to the Wet Method, the Dry Method is most often preferred for large-scale production, treating a large amount of filler in a relatively short time and generating relatively little mixed waste; however, it is more difficult to obtain uniform treatment with this method.
c) Spray Method
Spray the silane coupling agent on the high-temperature filler that was just taken out from the furnace. The method may omit dry procedure and make the process simplify, but pay attention to perfection and ignite.
 English
English 日本語
日本語 한국어
한국어 français
français Deutsch
Deutsch Español
Español italiano
italiano русский
русский português
português العربية
العربية tiếng việt
tiếng việt